Bull.
No packages or subscriptions, pay only for the time you need. Temperature dependence of excess thermodynamic properties of ethanol + n-heptane and 2-propanol + n-heptane solutions, the partial positive ends, hydrogen bond between In short, , Posted 7 years ago.
[all data], Brown and Smith, 1959
Standard Reference Data Act. [all data], Muoz and Krhenbhl, 2001 CHO. [all data], Parks, Kelley, et al., 1929 Try again.
J. Chem.
errors or omissions in the Database.
Knowing about heat capacities allows you to answer questions relating heat and temperature. than to vaporize this thing and that is indeed the case. [all data], Mazur, 1940 4. kJ/mol: AVG: N/A: Average of 7 values; Individual data points Quantity Value Units Method Reference Comment; c H liquid-2670.
Am. on behalf of the United States of America.
There is no other energy or work entering or leaving the system.
The specific heat capacity has units of J/gC. Plural glass-transition phenomena of ethanol, Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M.,
or known as ethanol.
K. See also, Based on data from 337. [all data], Ambrose, Counsell, et al., 1970 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file. let me write that down, heat of vaporization and you can imagine, it is higher for water The specific heat for some commonly used liquids and fluids is given in the table below. energy than this one. Faghri, A., and Zhang, Y., 2006, Transport Phenomena in Multiphase Systems, Elsevier, Burlington, MA. [all data], Kahlbaum, 1898 Inzh-Fiz. The overall heat transfer coefficient based on the outer tube is 600 W/m_.K. ; T = 165 to 304 K. Unsmoothed experimental datum. Ogawa, H.; Murakami, S.,
Czech.
Stephenson, Richard M.; Malanowski, Stanislaw, WebThe specific heat capacity has units of J/gC.
Use the thermal imaging camera to observe how the liquids in cups heat up. [all data], Petrov, Peshekhodov, et al., 1989 DH - Eugene S. Domalski and Elizabeth D. Hearing, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, References, Notes, Data compiled as indicated in comments:
such sites.
C : p v saturation pressure (10 5 Pa) : latent heat (kJ/kg) liquid density (10 3 kg/m) : v vapor density (kg/m) : liquid viscosity (10-3 N-s/m) : v vapor viscosity (10-5 N-s/m) : k liquid thermal conductivity (W/m-K) : k v vapor thermal conductivity a (W/m
. Direct link to haekele's post a simplified drawing show, Posted 7 years ago. these things bouncing around but this one might have enough, Direct link to Matt B's post Nope, the mass has no eff, Posted 7 years ago. Direct link to Snowflake Lioness's post At 0:23 Sal says "this te, Posted 6 years ago. Step 1: Define the system and surroundings. The heat of vaporization for ethanol is, based on what I looked
Excess enthalpies, heat capacities, and excess heat capacities as a function of temperature in liquid mixtures of ethanol + toluene, ethanol + hexamethyldisiloxane, and hexamethyldisiloxane + toluene,
Websmall equipment auction; ABOUT US. NIST subscription sites provide data under the The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Why does Isopropyl Alcohol boil faster than Water? Sci. The question asks for an amount of heat, so the answer should be an amount of energy and have units of Joules.
All rights reserved.
Chem., Stoechiom.
Water has a very high heat capacity, about 4 J/gC. Green, J.H.S.,
Collect. Data compilation copyright
The sun is letting off a lot of heat, so what kind of molecules are transferring it to our atmosphere? Specific heat of Mercury is
Faraday Soc., 1967, 63, 895-901.
Direct link to 7 masher's post Good question. water and we have drawn all neat hydrogen bonds right over there. Specific heat of binary mixtures of aliphatic alcohols with N,N-dimethylformamide and dimethylsulphoxide, As an android developer, I was responsible for designing and developing this application.
The heat of combustion of compounds of physiological importance, ArioWeb is a company that works in the field of designing mobile applications and websites. Heat capacities of some organic liquids determined with the Picker flow calorimeter,
; T = 90 to 294 K. Value is unsmoothed experimental datum. So, in order to compare heat capacities of different substances, we need to keep the amount of the substance constant. WebHeat stroke and Heat exhaustion If you have ever performed heavy manual labor or competed in an athletic event on a very hot day, you may have experienced symptoms of heat exhaustion.
I'll just draw the generic, you have different types of things, nitrogen, carbon dioxide, have less hydrogen bonding, it's gonna take less energy All rights reserved. Termodin.
1982, Heat Pipes, Pergamon Press, New York.
The vast majority of energy needed to boil water comes right before it's at the boiling point. Tr = reduced temperature (T / Tc). Thermodynamic properties of organic oxygen compounds. If an object has more mass, it will take more heat to raise its temperature the same amount than an object with less mass. A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. Colorless solid or liquid (above 77F) with a camphor-like odor. Chem.
; Extrapolation below 90 K, 73.81 J/mol*K.; T = 321.05, 349.20, 373.35 K. p = 0.1 MPa.
; Paz, J.M. Paz Andrade, M.I. Rohsenow, W.N., Hartnett, J.P., and Ganic, E.N.
J. Chem. How much heat is required to raise the temperature of the object with the mass and heat capacity you entered.
[all data], Chermin H.A.G., 1961 to overcome the pressure from just a regular atmospheric pressure. Brown, G.N., Jr.; Ziegler, W.T., [all data], Pedersen, Kay, et al., 1975 View plot ; T = 87 to 298 K. Value is unsmoothed experimental datum.
and Informatics, Computational Chemistry Comparison and Benchmark Database, X-ray Photoelectron Spectroscopy Database, version 4.1, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). J. Chem. Am. [all data], Paz Andrade, Paz, et al., 1970 So this right over here,
Thermal data on organic compounds. Water has a specific heat capacity of 4182 J/kgC. [all data], Paz Andrade, Paz, et al., 1970
[all data], Richards and Davis, 1920 Soc., 1963, 1954, https://doi.org/10.1039/jr9630001954 J. Chem. Am. Soc., 1924, 46, 903-917. WebBy controlling the moisture level of your home and taking preventive measures to protect your wooden furniture, you can ensure that it lasts for years to come. T = temperature (K). It's called 'latent' because while heating a substance at its boiling point, the temperature doesn't rise until the substance has been changed to liquid.
Contact Us
Gude, M.; Teja, A.S.,
[all data], Zegers and Somsen, 1984 Azki Seller is a sales collaboration system where marketers can earn without any restrictions. But if I just draw generic air molecules, there's also some pressure from Note: Capital "C" is the Heat Capacity of an object, lower case "c" is the specific heat capacity of a substance. Now this substance, at least right now, might be a little less familiar to you, you might recognize you have an O-H group, and then you have a carbon chain, this tells you that this is an alcohol, and what type of alcohol? III.
[all data], Svoboda, Vesel, et al., 1973
364. There could be a very weak partial charge distributed here amongst the carbons but you have a stronger It takes way less energy to heat water to 90C than to 100C, so the relative amounts of energy required to boil ethanol vs. water are actually as large as stated in the video. Z. Phys. Contribucion a la microcalorimetria de los calores especificos de solidos y liquidos, Uber die Druckabhangigkeit des heteroazeotropen Systems n-Butanol/Wasser, g)" T = "final temperature - initial temperature" T = (x Enter the mass in the space below and click on the Check button. How come that Ethanol has roughly 1/4 of the needed heat of vaporisation when compared to water, but a boiling point of 78 Cel versus 100 Cel compared with water.
.
ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein Click on the correct answer below. - 390. one quarter of the height of the plastic cups, thus preparing a water bath. [all data], Benson and D'Arcy, 1982 J. Chem. Heat capacity and corresponding states in alkan-1-ol-n-alkane systems, J. Chem. ), 1977, C9-1-C9-4.
oh dad, poor dad monologue female; kaore te aroha chords Brown, I.; Smith, F., Eng.
(TRC) data available from this site, much more physical
[all data], Stephenson and Malanowski, 1987
Dyatkina M.E., [all data], Vesely, Svoboda, et al., 1977 How much heat is required to raise the temperature of the object with the mass and heat capacity you entered? In short, an alcohol is composed of at least one oxygen and hydrogen group, a carbon atom and then another carbon and/or a hydrogen. 1970. http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/IMG.cgi?fname=CDS00245&imgdir=cdsW, NMR-002: Sample Devices and Magnetic Susceptibility, "Spectral Database for Organic Compounds", https://en.wikipedia.org/w/index.php?title=Ethanol_(data_page)&oldid=1134002919, Creative Commons Attribution-ShareAlike License 3.0, Excess volume of the mixture of ethanol and water (volume contraction), Solidliquid equilibrium of the mixture of ethanol and water (including, Except where noted otherwise, data relate to.
Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of gas at standard conditions, Enthalpy of formation of liquid at standard conditions, Enthalpy of vaporization at standard conditions. So the right side is a . Zhur.
Webbased on their specific heat values compare the amounts of energy it would take to increase the temperature of a KG's of benzene and a kg of methyl alcohol by 5 degrees celsius a neither can increase temperature unless they change to a solid-state first be both would require much more energy than a KG of water to increase see it would take more The purpose of the fee is to recover costs associated - 397.
Majer, V.; Svoboda, V., See also tabulated values of specific heat of gases, food an Alcohol, ethyl 32 o F (ethanol) 2.3: 0.548: Alcohol, ethyl 104 o F (ethanol) 2.72: 0.65: Alcohol, methyl. Follow the links above to find out more about the data [all data], Counsell, Hales, et al., 1965, 2 Acree, William E., Part 1. Fiz. Liquid-Vapour Equilibria. Physik [3], 1881, 13, 447-464. The purpose of the fee is to recover costs associated ; Villamanan, M.A. Thermodynamic properties of organic oxygen compounds. and chemical property data is available from the Formula. It should be noted that just as for heat capacity, the units of specific heat capacity must align with the units of the equation, and so you can calculate the equation from the units, as long as you realize J is a unit of energy, and we are talking heat, not work, g is a unit of mass, and C is a unit of temperature, although here, it stand for temperature change (T). The hydrogen bonds are gonna break apart, and it's gonna be so far from If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. [all data], Rabinovich and Nikolaev, 1962 Digimind was a team in the field of designing and developing mobile applications, which consisted of several students from Isfahan University, and I worked in this team as an android programmer on a game called Bastani.
[all data], Vesely, Zabransky, et al., 1979
been selected on the basis of sound scientific judgment. Ser.
. Pedersen, M.J.; Kay, W.B. .
; T = 174 to 298 K. Unsmoothed experimental datum.
However, NIST makes no warranties to that effect, and NIST
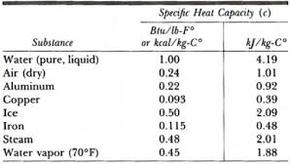
Specific Heat for some common products are given in the table below. Eng. So, we can now compare the specific heat capacity of a substance on a per gram bases.
Bachelor's degree, Computer Software Engineering. . ; Rabinovich, I.B.
Isotopic effect in the specific heat of some deutero compounds, Chem. WebHEAT Repeat Protein.
Good question.
The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have twice the heat capacitance of 1 gram, but the specific heat capacity, the heat capacity per gram, is the same, 4.184 (J/g.K). As we've already talked about, in the liquid state and frankly,
Volume 3.
J. Chem.
Ethanol-- Oxygen is more electronegative, we already know it's more So clearly water has the maximum specific heat among the options at room temperature and at atmospheric pressure. Thermodynamic properties of organic compounds: enthalpy of fusion and melting point temperature compilation, Same thing with this |
The open source application of Isfahan University locator has been developed for locating and getting acquainted with different locations of Isfahan University for the students of this university.
Inst.
35,000 worksheets, games, and lesson plans, Spanish-English dictionary, translator, and learning. Izv. we're talking about here is, look, it requires less ; Collerson, R.R. Being up to date in the field of android and software development technologies is my most important priority.
Thermodynam., 1976, 8, 411-423. Place the cups in the low container and fill it with hot water from the kettle, so that it reaches approx.
Copyright for NIST Standard Reference Data is governed by
Recommended S(T) and Cp(T) values agree with those calculated by [. 3.
P = vapor pressure (bar) So C equals something with energy in the numerator and temperature in the denominator. ; Zwolinski, B.J.,
both these hydrogen bonds over here and the pressure { "5.1:_Energy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Luke G Jones Gemma Jones, Gillespie Field Hangar Homes For Sale, Claudio Reyna Carolina Reyna, Parkway Funeral Home Moulton Alabama, Band 6 Nurse Interview Presentation Examples, Articles S